Health
Cervical Cancer: Prevention, Causes
By Laide Akinboade, Abuja
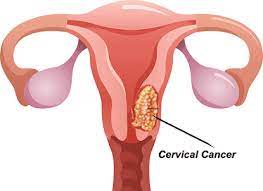
Cervical cancer is a type of cancer that occurs in the cells of the cervix, the lower part of the uterus that connects to the vagina.
Various strains of the human papillomavirus (HPV), a sexually transmitted infection, play a role in causing most cervical cancer.
When exposed to HPV, the body’s immune system typically prevents the virus from doing harm.
In a small percentage of people, however, the virus survives for years, contributing to the process that causes some cervical cells to become cancer cells.You can reduce your risk of developing cervical cancer by having screening tests and receiving a vaccine that protects against HPV infection.
Early-stage cervical cancer generally produces no signs or symptoms.
Signs and symptoms of more-advanced cervical cancer include:
Vaginal bleeding after intercourse, between periods or after menopause
Watery, bloody vaginal discharge that may be heavy and have a foul odor
Pelvic pain or pain during intercourse
Causes
Where cervical cancer begins
Cervical cancer begins when healthy cells in the cervix develop changes (mutations) in their DNA. A cell’s DNA contains the instructions that tell a cell what to do.
Healthy cells grow and multiply at a set rate, eventually dying at a set time. The mutations tell the cells to grow and multiply out of control, and they don’t die. The accumulating abnormal cells form a mass (tumor). Cancer cells invade nearby tissues and can break off from a tumor to spread (metastasize) elsewhere in the body.
It isn’t clear what causes cervical cancer, but it’s certain that HPV plays a role. HPV is very common, and most people with the virus never develop cancer. This means other factors, such as your environment or your lifestyle choices also determine whether you’ll develop cervical cancer.
Types of cervical cancer
The type of cervical cancer that you have helps determine your prognosis and treatment. The main types of cervical cancer are:
Squamous cell carcinoma. This type of cervical cancer begins in the thin, flat cells (squamous cells) lining the outer part of the cervix, which projects into the vagina. Most cervical cancers are squamous cell carcinomas.
Adenocarcinoma. This type of cervical cancer begins in the column-shaped glandular cells that line the cervical canal.
Sometimes, both types of cells are involved in cervical cancer. Very rarely, cancer occurs in other cells in the cervix.
Risk factors
Risk factors for cervical cancer include:
Many sexual partners. The greater your number of sexual partners — and the greater your partner’s number of sexual partners — the greater your chance of acquiring HPV.
Early sexual activity. Having sex at an early age increases your risk of HPV.
Other sexually transmitted infections (STIs). Having other STIs — such as chlamydia, gonorrhea, syphilis and HIV/AIDS — increases your risk of HPV.
A weakened immune system. You may be more likely to develop cervical cancer if your immune system is weakened by another health condition and you have HPV.
Smoking. Smoking is associated with squamous cell cervical cancer.
Exposure to miscarriage prevention drug. If your mother took a drug called diethylstilbestrol (DES) while pregnant in the 1950s, you may have an increased risk of a certain type of cervical cancer called clear cell adenocarcinoma.
Prevention
To reduce your risk of cervical cancer:
Ask your doctor about the HPV vaccine. Receiving a vaccination to prevent HPV infection may reduce your risk of cervical cancer and other HPV-related cancers. Ask your doctor whether an HPV vaccine is appropriate for you.
The HPV vaccine protects against the types of HPV that most often cause cervical, vaginal, and vulvar cancers.
HPV vaccination is recommended for preteens aged 11 to 12 years, but can be given starting at age 9.
HPV vaccine also is recommended for everyone through age 26 years, if they are not vaccinated already.
HPV vaccination is not recommended for everyone older than age 26 years. However, some adults age 27 through 45 years who are not already vaccinated may decide to get the HPV vaccine after speaking with their doctor about their risk for new HPV infections and the possible benefits of vaccination. HPV vaccination in this age range provides less benefit, as more people have already been exposed to HPV.
If vaccination is started before age 15, a two-dose schedule is recommended, with the doses given 6 to 12 months apart. For people who start the series after their 15th birthday, the vaccine is given in a series of three shots.
HPV vaccination prevents new HPV infections, but does not treat existing infections or diseases. This is why the HPV vaccine works best when given before any exposure to HPV. You should get screened for cervical cancer regularly, even if you received an HPV vaccine.
HPV vaccination is recommended for preteens aged 11 to 12 years, but can be given starting at age 9.
HPV vaccine also is recommended for everyone through age 26 years, if they are not vaccinated alread
HPV vaccination is not recommended for everyone older than age 26 years. However, some adults age 27 through 45 years who are not already vaccinated may decide to get the HPV vaccine after speaking with their doctor about their risk for new HPV infections and the possible benefits of vaccination. HPV vaccination in this age range provides less benefit, as more people have already been exposed to HPV.
If vaccination is started before age 15, a two-dose schedule is recommended, with the doses given 6 to 12 months apart. For people who start the series after their 15th birthday, the vaccine is given in a series of three shots.
HPV vaccination prevents new HPV infections, but does not treat existing infections or diseases. This is why the HPV vaccine works best when given before any exposure to HPV. You should get screened for cervical cancer regularly, even if you received an HPV vaccine.
Have routine Pap tests. Pap tests can detect precancerous conditions of the cervix, so they can be monitored or treated in order to prevent cervical cancer. Most medical organizations suggest beginning routine Pap tests at age 21 and repeating them every few years.
Practice safe sex. Reduce your risk of cervical cancer by taking measures to prevent sexually transmitted infections, such as using a condom every time you have sex and limiting the number of sexual partners you have.
Don’t smoke. If you don’t smoke, don’t start. If you do smoke, talk to your doctor about strategies to help you quit.
Health
WHO Approves 2 New Vaccines to Protect Infants From RSV

The World Health Organization (WHO), on Friday, issued recommendations for two new immunisation tools to protect infants from Respiratory Syncytial Virus (RSV)They included a maternal vaccine, administered to pregnant women in their third trimester to protect their newborns.The other was a long-acting antibody injection for infants, which begins to protect within a week of administration and lasts for at least five months.
According to WHO, RSV is the leading cause of acute lower respiratory infections in children globally. It causes around 100,000 deaths and 3.6 million hospitalisations each year among children under the age of five, while infants under six months are most at risk.Alarmingly, 97 per cent of these deaths occur in low and middle-income countries, according to WHO.Although RSV can infect people of all ages, “it is especially harmful to infants, particularly those born prematurely,” a WHO official, Kate O’Brien, said.O’Brien added that around half of all RSV-related deaths occurred in babies younger than six months.Considering the global burden of severe RSV illness in infants, WHO recommended that all countries adopt either the maternal vaccine or the antibody injection as part of their national immunisation strategies.“These RSV immunisation products can transform the fight against severe RSV disease, dramatically reduce hospitalisations and deaths, and ultimately save many infant lives worldwide,” O’Brien said. (NAN)Health
UNICEF Promotes Menstrual Hygiene for Girls

The United Nations Children’s Fund (UNICEF) has encouraged girls to embrace menstruation with pride and confidence, recognizing themselves as vital contributors to humanity’s sustainability.
Mrs Aderonke Akinwole, Social and Behavioural Change Specialist at UNICEF, gave the advice during an event on Wednesday organised with the Nigeria Girls’ Guild and Lagos State Primary Health Care Board.
The event was held to commemorate Menstrual Hygiene Day (MHDay) and was attended by students from both public and private schools across Lagos.
With the theme ‘Together for a Period Friendly World,’ the event aimed to raise awareness and promote dignity in menstrual hygiene.
“When a girl begins menstruation, it should be celebrated. It signifies her transition into womanhood and her ability to sustain life.
“They should be proud, and seek accurate, helpful information to remain safe, clean, and healthy during their period,” Akinwole said.
She emphasised that girls must not feel ashamed, as menstruation is a natural part of womanhood and a symbol of female dignity.
She urged the state government to increase sensitisation efforts and include menstrual hygiene education in school curricula, religious settings, and community platforms.
Akinwole also warned against stigmatisation, especially from boys, and called for boys to be educated to respect menstruation as part of girls’ lives.
“Girls should understand the menstrual cycle even before it starts. This should be part of health education in schools, churches, mosques, and communities,” she said.
She explained that girls need awareness on menstrual hygiene management and should know how to prepare for their periods in a healthy, informed way.
Mrs Honfor Adesola, Director of Education at Lagos State Primary Education Board, commended UNICEF’s support in promoting menstrual hygiene and addressing issues affecting girls.
Adesola highlighted that maintaining menstrual hygiene is vital in preventing infections and ensuring comfort throughout the menstrual cycle.
She noted that the event also helped to raise awareness about the Human Papillomavirus (HPV) vaccine available free in health centres across Lagos.
“We’re here to mark MHDay and to engage girls on HPV awareness. The state government has provided the vaccine, and sensitisation must continue,” she explained.
She encouraged girls to discuss the HPV vaccine with their parents to gain consent, ensuring protection against cervical cancer.
“The vaccine is safe, effective, and accessible in state facilities for girls aged nine to fourteen, but many have not yet been vaccinated,” she added.
Meanwhile, Ethagah Divine, Head Girl of New Estate Baptist Secondary School, Surulere, called on NGOs to provide sanitary pads for girls.
She urged more campaigns and rallies to distribute free menstrual products, like UNICEF did, to promote hygiene and dignity during menstruation.
Miss Emmanuella Azubuike, a student of the same school, expressed gratitude to UNICEF and partners for the impactful menstrual hygiene awareness event.
“This programme has expanded my knowledge on menstrual hygiene and HPV. More NGOs should support these campaigns to reach and educate more young girls,” she said. (NAN)
Health
Soludo’s Wife Establishes Pad Banks in 300 schools

Wife of Anambra State Governor, Dr Nonye Soludo, says she has established pad banks in 300 schools across the state as part of her pet project, Healthy Living Initiative.
Mrs Soludo disclosed this in a message in Awka on Wednesday to mark the 2025 World Menstrual Hygiene Day.
She said that the initiative was her own approach to helping school girls whose academic focus could be affected during menstruation and related emergencies.
Mrs Soludo stressed the need to provide immediate solutions for menstrual emergencies in schools, so that girls caught off guard could confidently rely on the pad banks.
“Official data say that an estimated 37 million women and girls in Nigeria are unable to afford sanitary pads and only rely on unhygienic alternatives.
“The data reinforce World Health Organisation and United Nations Children’s Fund finding that poor water, sanitation and hygiene infrastructure hinders safe and dignified menstruation for women and girls.
“Other data say that only two in five schools globally offer menstrual health education and just one in three have bins for menstrual waste.
“These figures challenge key stakeholders to find practical solutions to address the root of the problem while the situation remains reversible.”
She called for intensified campaign to reach more women and girls currently facing menstrual hygiene challenges.
The governor’s wife noted that the growing number of women, especially girls, in urgent need of menstrual support makes it essential for stakeholders to re-strategise their campaign approach.
According to her, menstrual health remains the right of every girl-child.
She encouraged girls at the designated schools participating in the pad bank project to use the supplies with confidence.
Mrs Soludo assured them that her NGO was fully committed to restocking any of the pad banks that run out of sanitary products.(NAN)




















